Smart Injectable Devices
While most of the earlier platforms discussed have focused on oral medication therapies, there is ample room to use sensors and other digital technologies to assess adherence in different drug delivery devices. Injectables make up a large part of special medications amongst patients, especially those patients that use insulin or biologic therapies. With that in mind, the following companies have created smart injectable devices that are standalone or attach directly to an injected drug product to track adherence. I am not including timers or low tech solutions at this time and only those that integrate the data widely and can be accessed and shared with clinicians for medication management.
Note: One company worth mentioning that kinda fits here is HealthBeacon, a Dublin based start-up that has a Smart Bin that can track adherence with injectables when the needle/syringe is disposed. Lastly, I have not included smart pens from pharmaceutical companies at this time (Such as what Eli Lilly is currently doing), I may come back and update this after those products get further along and enter B2B or B2C markets.
Insulog
The Insulog was launched on crowdfunding site IndieGoGo, raising more than $45,000. The snap-on device, which costs $199.99, goes on the end of the insulin pen and keeps track of how much insulin is injected. This information is paired with a mobile app and then serves as an electronic diary. The app can also incorporate data from other devices and data such as blood glucose levels collected from another platform.
Research
No identified research or publications.
Companion Medical
San Diego-California-based Companion Medical's sleek InPen uses insulin cartridges. The InPen, which costs $665, offers data reporting, a built-in temperature sensor, a dose calculator, and a dose reminder. All this is paired with an app for the patient to access. The InPen has received FDA approval and is only available in the United States. Patients need a prescription for the InPen to get it via mail, though some insurance plans may cover it.
Research
No identified research or publications.
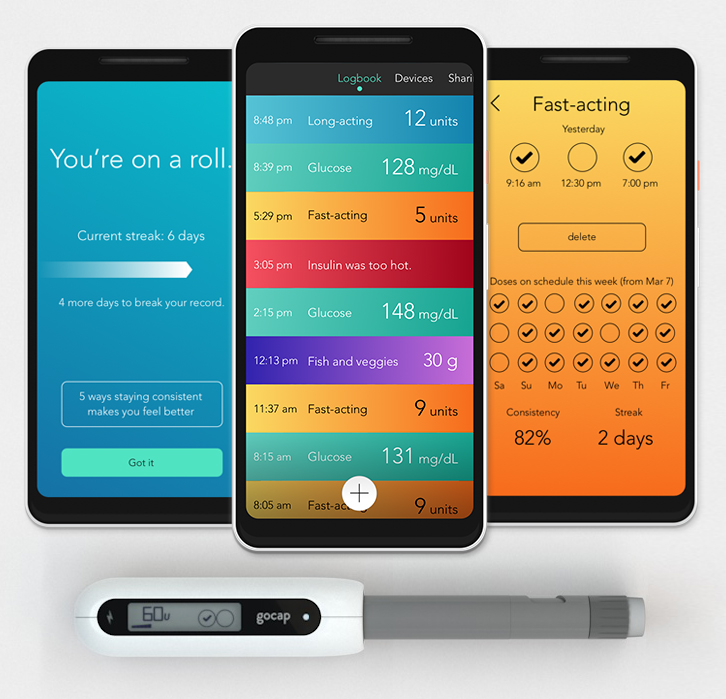
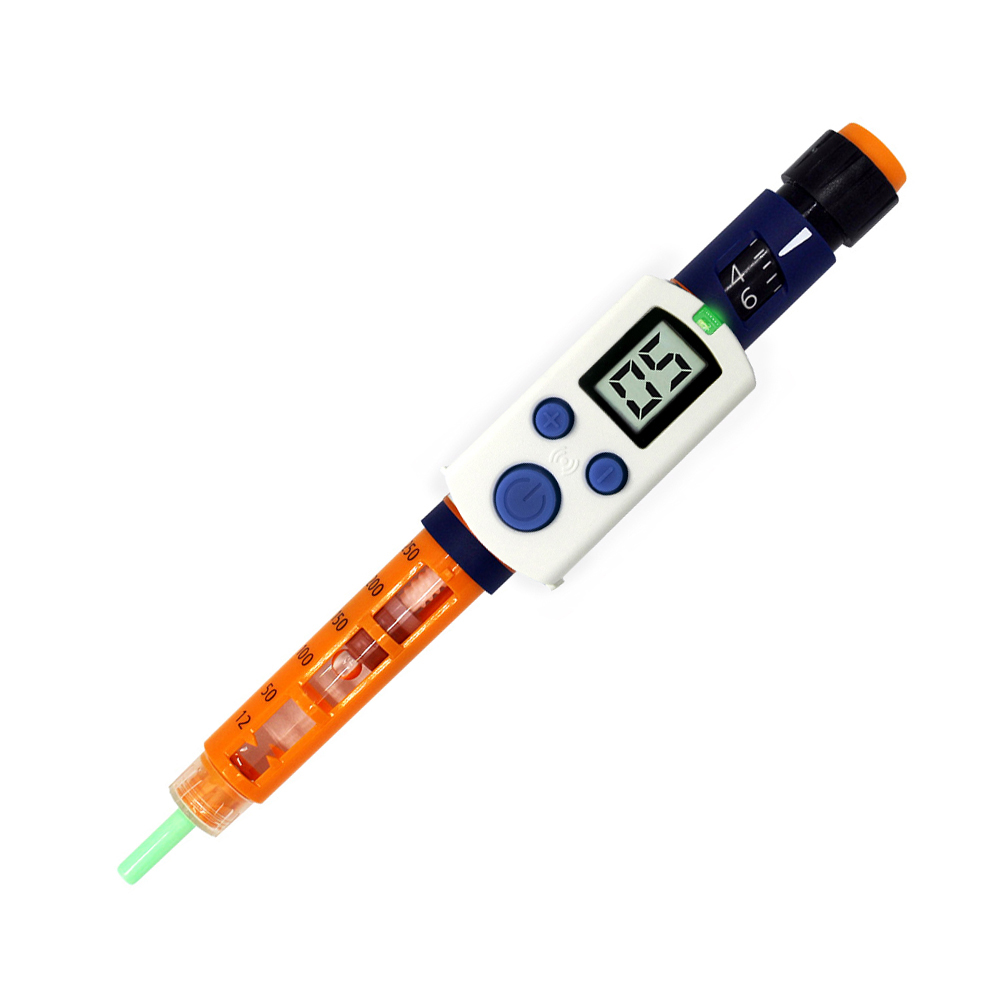
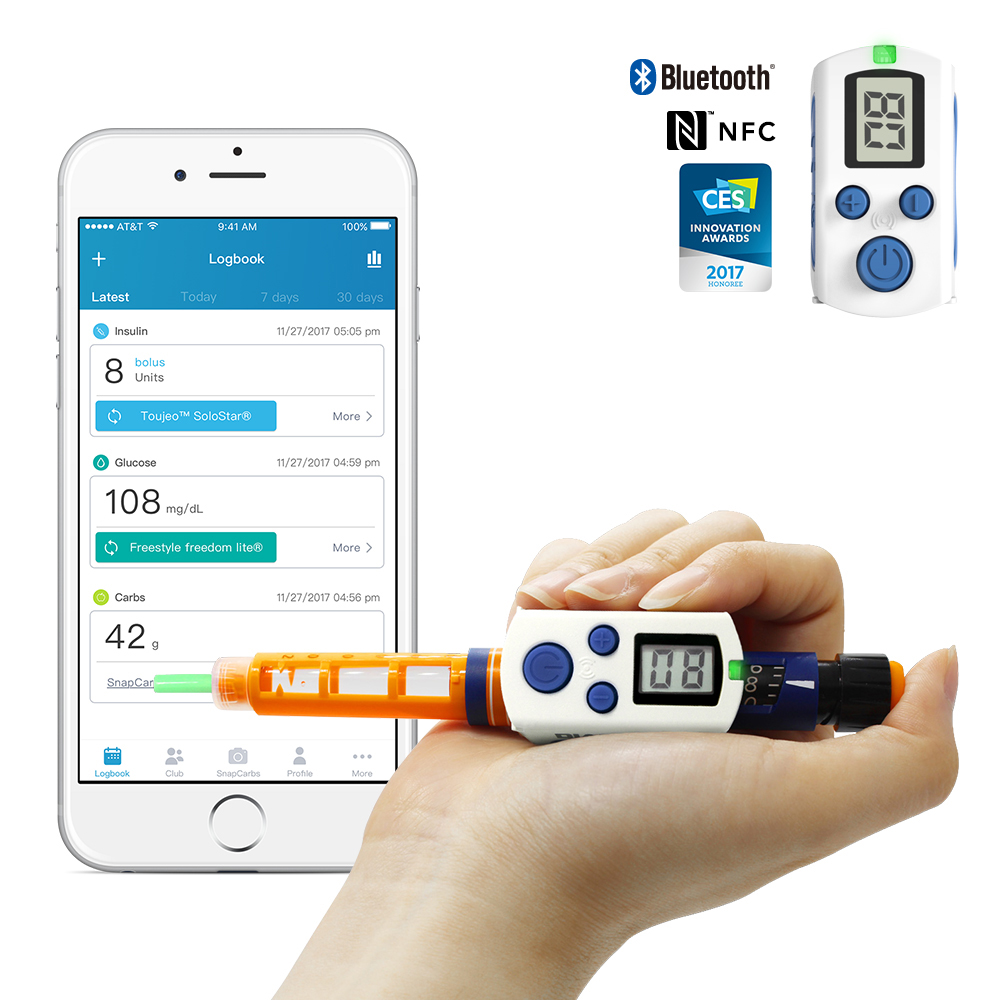
Gocap
Common Sensing of Cambridge, Massachusetts, is focused on creating smart devices, and the GoCap is its first product. The smart cap goes on the insulin pen, replacing the existing cap, like the InPen. The GoCap also tracks insulin dose and temperature, provides reminders and reports, and has an app to keep track of everything. The GoCap also keeps track of the amount of insulin in a pen. In addition, it has a sensor that monitors how much insulin is depleted out of the clear side of the pen that it covers. The GoCap is in Beta testing.
Research
A study was listed on ClinicalTrials.gov
SmartPlus
German based company that produces the SmartPlus Insulin Pen available in the EU under the name Pendiq. Insulin cartridges are inserted into the device, and an LCD screen tracks units of insulin administered which is uploaded onto a cloud platform and syncs with a mobile app.
Research
No studies or publications identified.
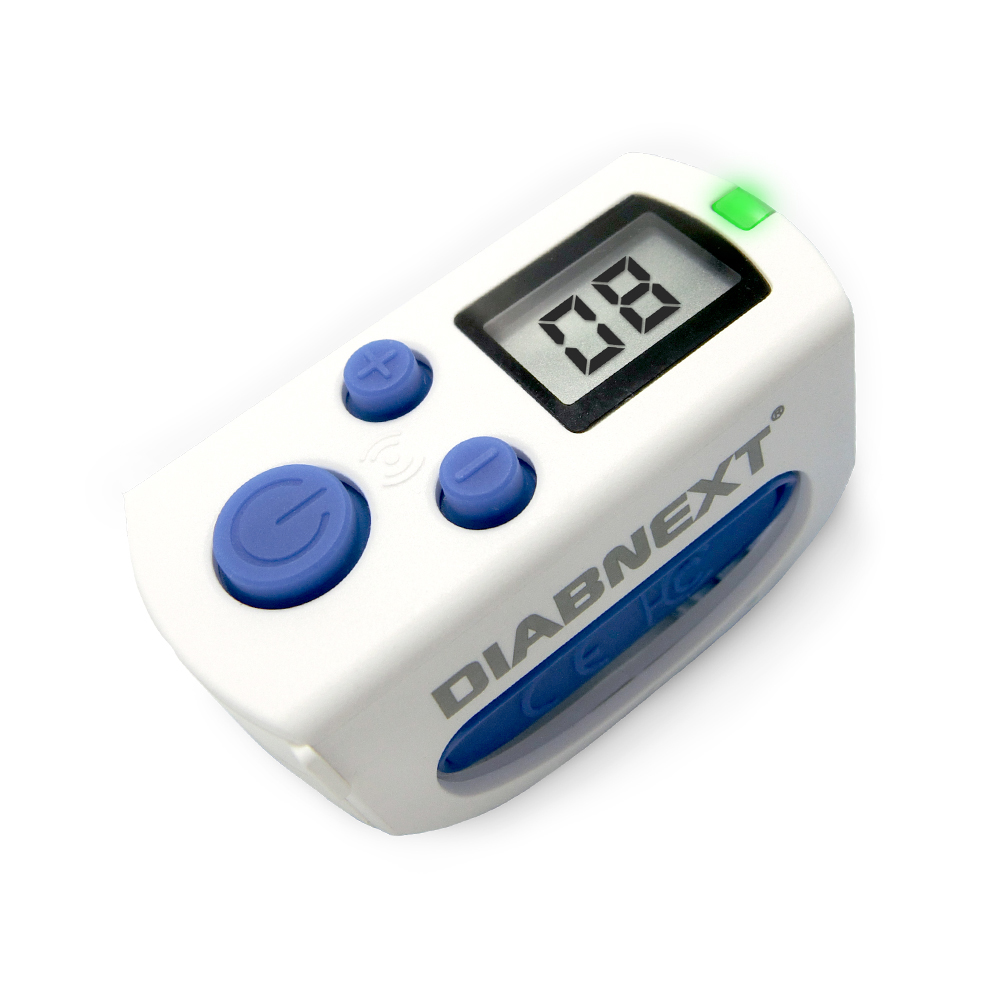
DiabNext
DiabNext produces a line of digital health devices in the diabetes space, including a smart pill monitor, a carb counter, and blood glucose monitor. However, I have put them here due to their smart insulin monitor called Clipsulin, which as the name infers, clips onto and insulin pen. As each unit is dispensed, the device syncs with their mobile app (DiabNext) to keep track of insulin administered.
Research
No research noted at this time.
Emperra
Based in Germany, the company makes a smart insulin pen that takes insulin cartridges, but it runs on the EYSTA platform, which allows those with a mobile phone to have an application to track data. Those who do not have mobile phones receive a home device that can export that data to an online cloud database for providers to have access to and use in patient care. It is a novel system and seems more clinically focused on patient management than the other products. The product is not available in the United States, however.
Research
Their webpage lists more on the science of their product.